肥胖症是美国第二大可预防死亡原因。它与多种合并症相关,包括2型糖尿病(T2D)、代谢功能障碍相关肝病(MASLD,之前称为非酒精性脂肪肝病,NAFLD)、心血管疾病和癌症。最初为治疗T2D而开发的胰高血糖素样肽1(GLP-1)、葡萄糖依赖性胰岛素促进多肽(GIP)和胰高血糖素受体激动剂在肥胖症治疗中发现了新的治疗用途。
Cayman中国区总代理,艾美捷科技,提供这些仅供研究使用的试剂,以支持2型糖尿病和肥胖症等代谢性疾病的研究。本文探讨了这些试剂背后的科学。
胰岛素和胰高血糖素调节血糖稳态
调节血糖水平主要由胰腺释放的激素:胰岛素和胰高血糖素驱动。这两种激素作用相反,共同调节血糖稳态。
胰岛素在血糖水平高(高血糖)时释放,通过向细胞发出信号吸收血液中的葡萄糖以供能量或储存,从而降低血糖水平。胰高血糖素在血糖水平低(低血糖)时释放。它刺激肝脏通过糖原分解增加葡萄糖产生,从而提高血糖水平。
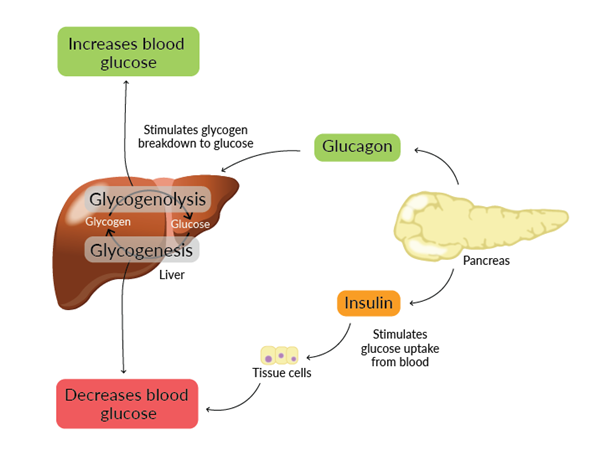
图1. 葡萄糖稳态中胰岛素和胰高血糖素途径的总结。
GLP-1和GIP通过刺激餐后胰岛素释放来调节血糖水平
餐后血糖水平通常会上升,禁食时下降。餐后,葡萄糖——细胞进行无数生物过程所需的关键能量来源——被释放到血液中,增加血糖水平。
为了刺激餐后胰岛素的分泌,身体利用两种在肠道产生的肠促胰岛素激素:胰高血糖素样肽1(GLP-1)和葡萄糖依赖性胰岛素促进多肽(GIP)。这些激素在餐后释放,并刺激胰腺分泌胰岛素,向细胞发出信号吸收葡萄糖以供能量或储存,减少餐后血糖水平的增加。
GLP-1调节食欲和食物摄入量
除了刺激胰岛素产生外,GLP-1(而不是GIP)还减缓胃排空,降低肠道运动性,减少食物摄入和食欲,并通过复杂的中枢和外周途径刺激胰高血糖素依赖的能量消耗。这些效应共同促进减重,使GLP-1受体(GLP-1R)激动剂成为肥胖症的有价值方法。

图2. GLP-1和GIP在葡萄糖稳态和体重减轻中的作用的总结
肥胖症研究中的肠促胰岛素基础疗法
单一激动剂
塞美格鲁肽是一种GLP-1R激动剂,是一种GLP-1的肽类似物,用于促进减重。研究表明,与仅改变生活方式(如减少热量摄入和增加体力活动)相比,它可以帮助肥胖个体实现更大的减重效果。
其他基于肽的GLP-1R激动剂,如利西那肽、利拉鲁肽、度拉糖肽和艾塞那肽,它们在分子结构、大小、药理学、效力和安全性上有所不同,根据个体情况提供各种优势。
非肽类疗法可能比基于肽的疗法提供优势。基于肽的疗法通常是大分子量化合物,需要注射,而非肽类GLP-1R激动剂,如orforglipron,或正向别构调节剂,如V-0219,可以作为口服制剂。这些非肽GLP-1R激动剂正在进行临床试验。
双重激动剂
曲普瑞肽是一种首创的双重GLP-1R和GIPR激动剂。虽然GIP单独似乎不会延迟胃排空或改变食物摄入和食欲,但由于尚不清楚的原因,GLP-1和GIP受体(GIPR)激动剂协同作用,与单独使用GLP-1R激动剂相比提供更大的益处。这些双重GLP-1R和GIPR激动剂,俗称“双促胰岛素”,似乎也因为它们的双重作用机制而减少副作用。这些双重激动剂的优势激发了进一步研究针对多重作用机制的疗法。
三重激动剂
一种新的方法是通过针对三个激素受体。胰高血糖素受体(GCGR)拮抗剂最初作为T2D疗法被追求,基于它们会减弱葡萄糖产生并促进胰岛素分泌的想法。然而,由于与肝脂肪变性发展相关的MASLD安全问题,这些药物开发努力不再受青睐。
然而,尽管GCGR激动剂具有高血糖效应,但它们通过刺激热生成促进饱腹感和增加能量消耗,使它们成为研究肥胖症的潜在宝贵补充。GCGR激动作用的高血糖效应可以通过包括刺激胰岛素分泌的制剂来抵消,从而降低血糖水平,使GLP-1R和/或GIPR激动剂的多激动剂制剂成为肥胖症的新疗法。事实上,retatrutide,一种三重GLP-1R、GIPR和GCGR激动剂,在临床试验中显示出前景。
| GLP-1R Agonists | GIPR Agonists | GCGR Agonists | |
| SemaglutideA peptide analog |
| ||
| LixisenatideA peptide analog |
| ||
| DulaglutideA peptide analog |
| ||
| ExendinA peptide analog |
| ||
| OrforglipronA non-peptide agonist |
| ||
| V-0219A non-peptide positive allosteric modulator |
| ||
| LSN3318839A non-peptide positive allosteric modulator |
| ||
| Taspoglutide (acetate)A peptide analog |
| ||
| LiraglutideA peptide analog |
| ||
| TirzepatideA peptide analog |
|
| |
| BamadutideA peptide analog |
|
| |
| Cotadutide (acetate)A peptide analog |
|
| |
| SurvodutideA peptide analog |
|
| |
| MazdutideA peptide analog |
|
| |
| RetatrutideA peptide analog |
|
|
|
| SAR441255 (sodium salt)A peptide analog |
|
|
|
| These products are for scientific research use only. | |||
未来方向
此外,这些重新用于T2D治疗的许多疗法不仅在肥胖症中被探索,还在与肥胖症相关的其他疾病中被探索,包括心力衰竭和心血管疾病。这些相同的制剂还显示出治疗其他疾病,如物质使用障碍和神经退行性疾病如阿尔茨海默病和帕金森病的潜在益处。
以上产品仅供科学研究使用。
超900+糖尿病研究相关研究试剂,欢迎垂询Cayman中国区总代理,艾美捷科技:

参考文献
1. Wang, Y., Beydoun, M.A., Min, J., et al. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int. J. Epidemiol.49(3), 810-823 (2020).
2. Zhang, X., Ha, S., Lau, H. C.-H., et al. Excess body weight: Novel insights into its roles in obesity comorbidities. Semin. Cancer Biol.92, 16-27 (2023).
3. R?der, P.V., Wu, B., Liu, Y., et al. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med.48(3), e219 (2016).
4. Nakrani, M.N., Wineland, R.H., and Anjum, F. Physiology, glucose metabolism. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2024). Available from: https://www.ncbi.nlm.nih.gov/books/NBK560599/
5. Baggio, L.L. and Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology132(6), 2131-2157 (2007).
6. Nauck, M.A., Quast, D.R., Wefers, J. et al. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes. Metab.23(Suppl 3), 5-29 (2021).
7. González-García, I., Milbank, E., Diéguez, C., et al. Glucagon, GLP-1 and thermogenesis. Int. J. Mol. Sci.20(14), 3445 (2019).
8. Holst, J.J. and Rosenkilde, M.M. GIP as a therapeutic target in diabetes and obesity: Insight from incretin co-agonists. J. Clin. Endocrinol. Metab.105(8), e2710-e2716 (2020).
9. Edholm, T., Degerblad, M., Gryb?ck, P. et al. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil.22(11), 1191-1200, e315 (2010).
10. Wadden, T.A., Chao, A.M., Moore, M., et al. The role of lifestyle modification with second-generation anti-obesity medications: Comparisons, questions, and clinical opportunities. Curr. Obes. Rep.12(4), 453-473 (2023).
11. Almandoz, J.P., Lingvay, I., Morales, J., et al. Switching between glucagon-like peptide-1 receptor agonists: Rationale and practical guidance. Clin. Diabetes38(4), 390-402 (2020).
12. Malik, F. and Li, Z. Non‐peptide agonists and positive allosteric modulators of glucagon‐like peptide‐1 receptors: Alternative approaches for treatment of type 2 diabetes. Br. J. Pharmacol.179(4), 511-525 (2022).
13. Wharton, S., Blevins, T., Connery, L., et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med.389(10), 877-888 (2023).
14. Decara, J.M., Vázquez-Villa, H., Brea, J., et al. Discovery of V-0219: A small-molecule positive allosteric modulator of the glucagon-like peptide-1 receptor toward oral treatment for "diabesity"'. J. Med. Chem.65(7), 5449-5461 (2022).
15. Lin, F., Yu, B., Ling, B., et al. Weight loss efficiency and safety of tirzepatide: A systematic review. PLoS One18(5), e0285197 (2023).
16. Hope, D.C.D., Vincent, M.L., and Tan, T.M.M. Striking the balance: GLP-1/glucagon co-agonism as a treatment strategy for obesity. Front. Endocrinol.(Lausanne)12, 735019 (2021).
17. Novikoff, A. and Müller, T.D. The molecular pharmacology of glucagon agonists in diabetes and obesity. Peptides165, 171003 (2023).
18. Naeem, M., Imran, L., and Banatwala, U.E.S.S. Unleashing the power of retatrutide: A possible triumph over obesity and overweight: A correspondence. Health Sci. Rep.7(2), e1864 (2024).
19. Kosiborod, M.N, Abildstr?m, S.Z., Borlaug, B.A., et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med.389(12), 1069-1084 (2023).
20. Husain, M., Bain, S.C., Jeppesen, O.K., et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes. Metab.22(3), 442-451 (2020).
21. Ryan, D.H., Lingvay, I., Colhoun, H.M., et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am. Heart J.229, 61-69 (2020).
22. Edwards, K.L. and Minze, M.G. Dulaglutide: An evidence-based review of its potential in the treatment of type 2 diabetes. Core Evid.10, 11-21 (2015).
23. Thomsen, M., Holst, J.J., Molander, A., et al. Effects of glucagon-like peptide 1 analogs on alcohol intake in alcohol-preferring vervet monkeys. Psychopharmacology(Berl.)236(2), 603-611 (2019).
24. Yammine, L., Green, C.E., Kosten, T.R., et al. Exenatide adjunct to nicotine patch facilitates smoking cessation and may reduce post-cessation weight gain: A pilot randomized controlled trial. Nicotine Tob. Res.23(10), 1682-1690 (2021).
25. Leggio, L., Hendershot, C.S., Farokhnia, M., et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med.29(12), 2993-2995 (2023).
26. Mahapatra, M.K., Karuppasamy, M., and Sahoo, B.M. Therapeutic potential of semaglutide, a newer GLP-1 receptor agonist, in abating obesity, non-alcoholic steatohepatitis and neurodegenerative diseases: A narrative review. Pharm. Res.39(6), 1233-1248 (2022).
欢迎垂询,Cayman 中国区总代理,艾美捷科技,www.amyjet.com,了解更多产品详情,开启糖尿病与肥胖研究的新篇章。





 经营性网站备案信息
经营性网站备案信息 鄂公网安备42018502007175
鄂公网安备42018502007175